Many places in space are too far away to learn about them by sending spacecraft there. So they cannot be examined directly but instead, we can learn about them by analyzing their emitted light.
Due to quantum mechanics, each molecule has a set of characteristic transition lines that uniquely identify it. When these transition lines are found in the emitted spectrum, we can be sure that the respective molecule appears in the observed object.
However, to identify molecules in space, we first have to understand their characteristic patterns in the laboratory. We do so by measuring the rotational spectrum of the molecules in our experiment and then fitting quantum mechanical models to them. These models can then be used by astronomers to identify the molecules in space and also to infer the physical conditions of the corresponding regions in space. For example, the temperature can be deduced from intensity relations, the pressure from the lineshape, and the molecule’s abundance can be inferred from its intensity.
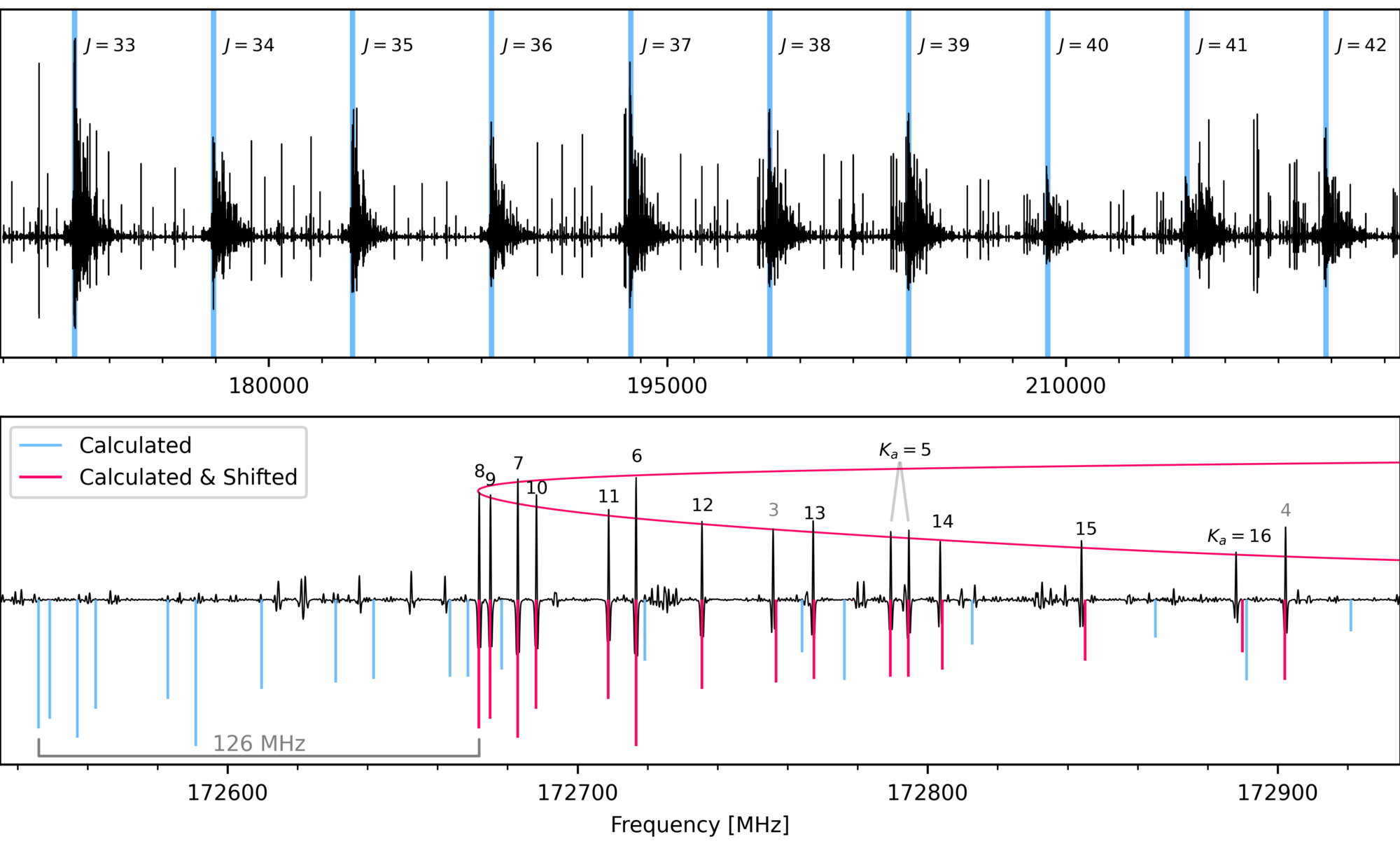
Here, we measured the pure rotational spectrum of phosphabutyne (C2H5CP) for the first time and analyzed its vibrational ground state as well as its three singly 13C-substituted isotopologues. This will allow astronomers to search for phosphabutyne in space and determine the prevailing conditions of the corresponding regions. The figure shows a section of the measured broadband spectrum on top, highlighting the pattern repeating with the total angular momentum quantum number J, while the zoom-in on the bottom highlights the very good agreement between the calculated and measured spectrum, especially when applying a small shift of 126 MHz.
This work was performed in collaboration with Jean-Claude Guillemin (University Rennes) and Michael E. Harding (KIT).